42 label these groups of the periodic table
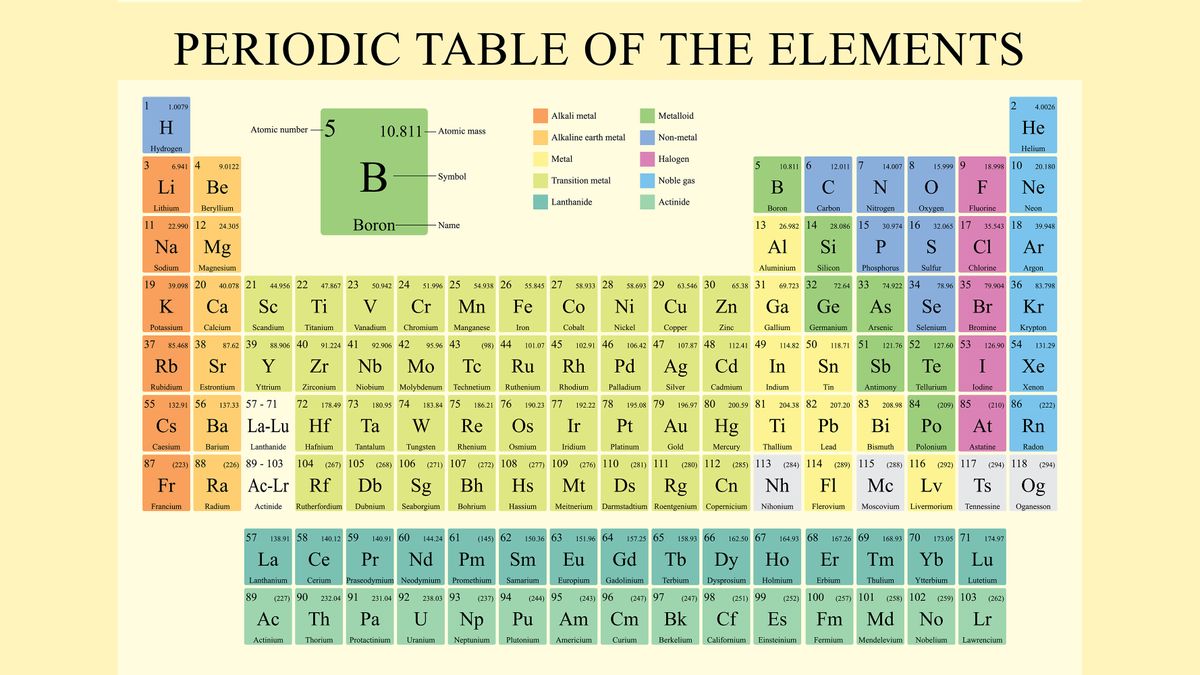
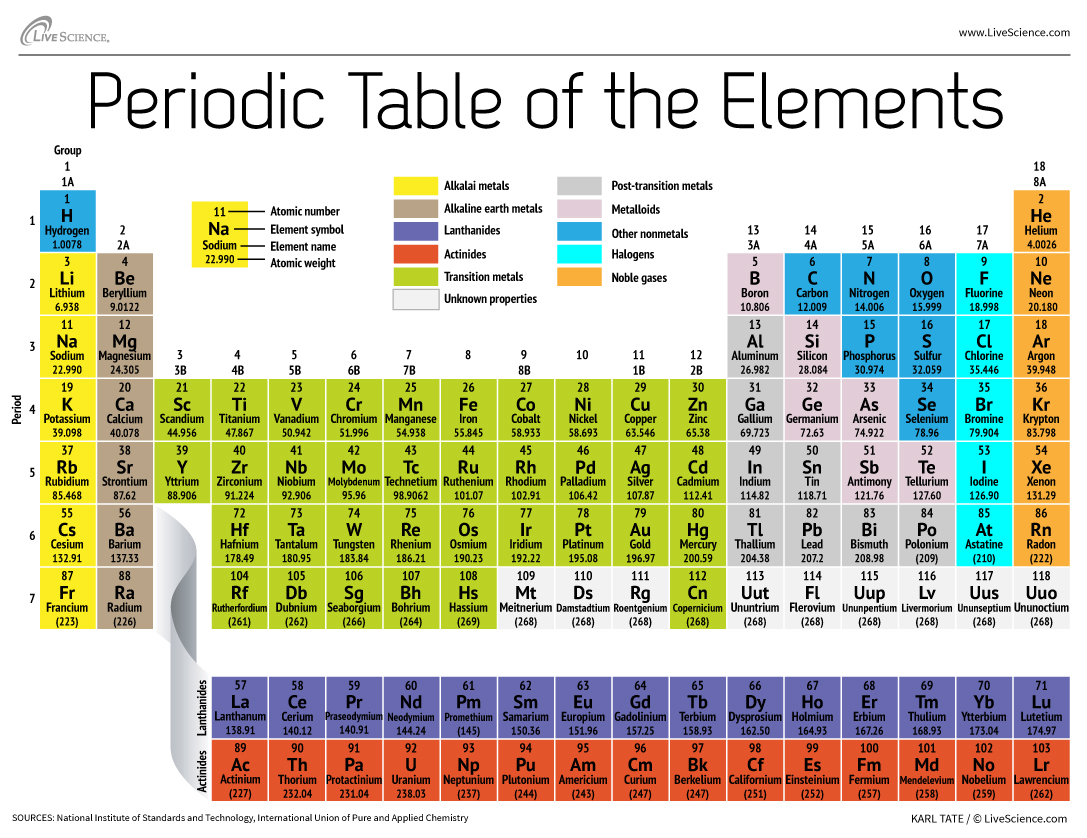
Periodic Table of Elements and Chemistry The periodic table we use today is based on the one devised and published by Dmitri Mendeleev in 1869. Mendeleev found he could arrange the 65 elements then known in a grid or table so that each element had: 1. A higher atomic weight than the one on its left. For example, magnesium (atomic weight 24.3) is placed to the right of sodium (atomic weight 23.0): The True Basis of … Metals on the Periodic Table: Definition & Reactivity 13.10.2021 · This periodic table groups elements according to type: metal (blue), nonmetal (yellow), or metalloid (red). All of the metals are grouped together on the left …
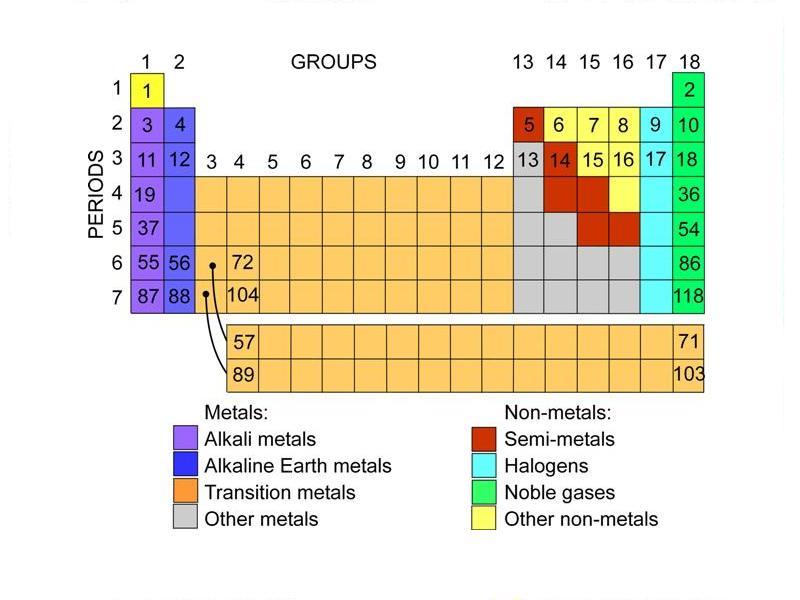
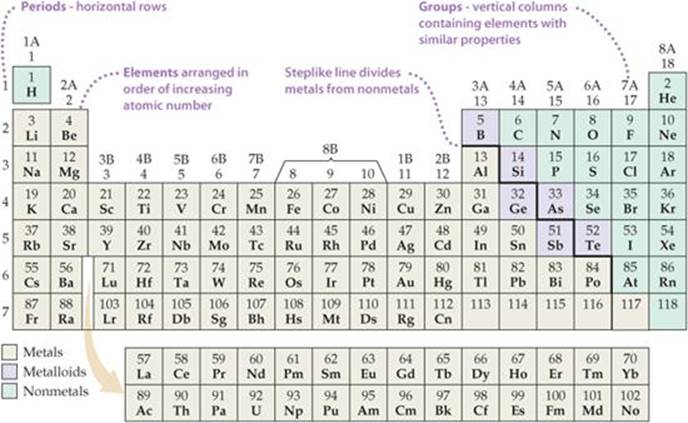
The Parts of the Periodic Table - angelo.edu The Periodic Table of the Elements summarizes a great deal of information about the properties of the chemical elements. Groups: the vertical columns on the table. These define a "family" of elements which have similar chemical properties. Periods: the horizontal rows on the table, with the elements arranged in order of increasing atomic number.
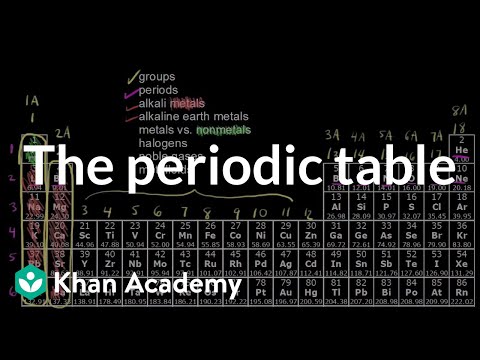
Label these groups of the periodic table
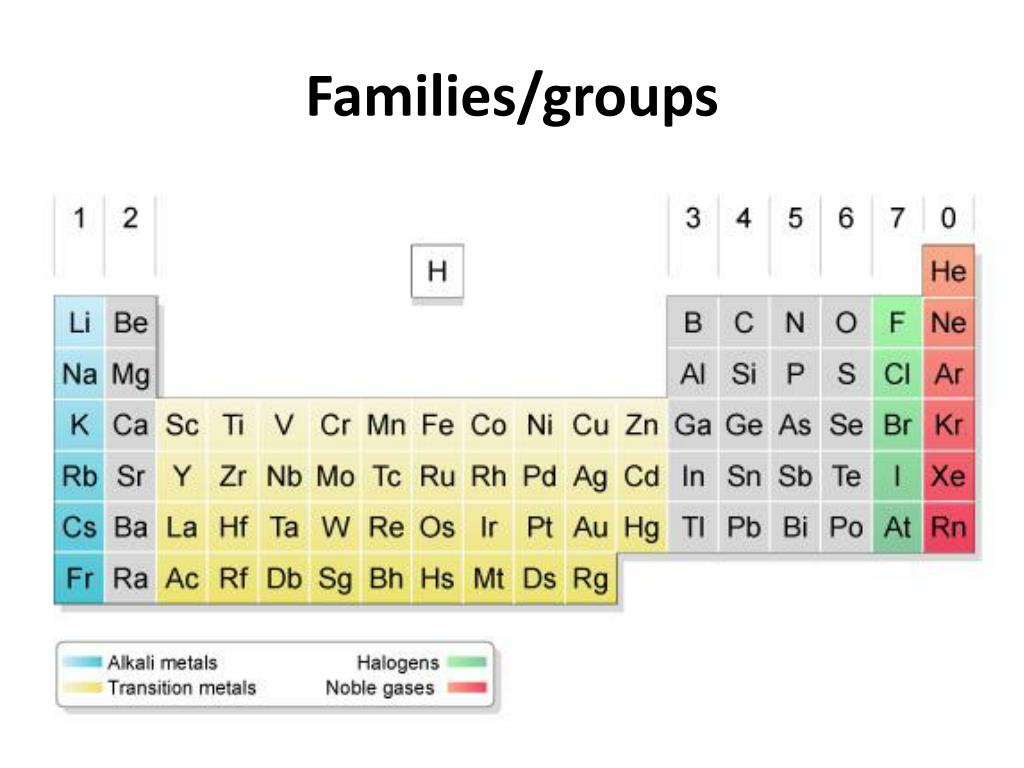
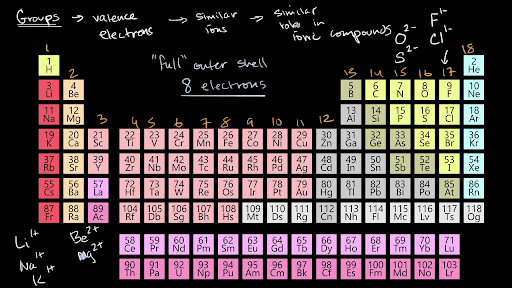
Periodic table Groups Explained !! (With 1-18 Group Names) Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li) Sodium (Na) Potassium (K) Rubidium (Rb) Cesium (Cs) Francium (Fr) For detailed information on Alkali metals, read the Ultimate guide on Alkali metals of periodic table. Groups of the periodic table (video) | Khan Academy The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups. The elements in each group have the same number of valence electrons. As a result, elements in the same group often display similar properties and reactivity. en.wikipedia.org › wiki › Functional_groupFunctional group - Wikipedia Table of common functional groups. The following is a list of common functional groups. In the formulas, the symbols R and R' usually denote an attached hydrogen, or a hydrocarbon side chain of any length, but may sometimes refer to any group of atoms.
Label these groups of the periodic table. Chemical Elements.com - An Interactive Periodic Table of the … An up-to-date periodic table with detailed but easy to understand information. Home About This Site Comments Help Links Window Version. Show Table With: Name Atomic Number Atomic Mass Electron Configuration Number of Neutrons Melting Point Boiling Point Date of Discovery Crystal Structure. Element Groups: Alkali Metals Alkaline Earth Metals Transition Metals Other … The Periodic Table Flashcards | Quizlet The horizontal rows in the periodic table are called. periods. How many periods are there in the periodic table. 7. What is the vertical row called in the period table. group. What are the names of the three classes of elements. metal, nonmetal, metalloids. Group 1 (but not H) forms a base when reacting with water. Properties of Periodic Table of Element Groups - ThoughtCo 01.04.2016 · This interactive periodic table of element groups arranges the chemical elements according to periodicity or common properties. Menu. Home. Science, Tech, Math Science Math Social Sciences Computer Science Animals & Nature Humanities History & Culture Visual Arts Literature English Geography Philosophy Issues Languages English as a Second Language … Labeled Periodic Table of Elements with Names - Science Struck Helium, Neon, Argon, Krypton, Xenon and Radon are six noble gases, found in the periodic table. Given below is a labeled periodic table of elements with their names and atomic number. Hold the mouse on each atomic symbol to know the name of the chemical element. Periodic Table Key for the Periodic Table
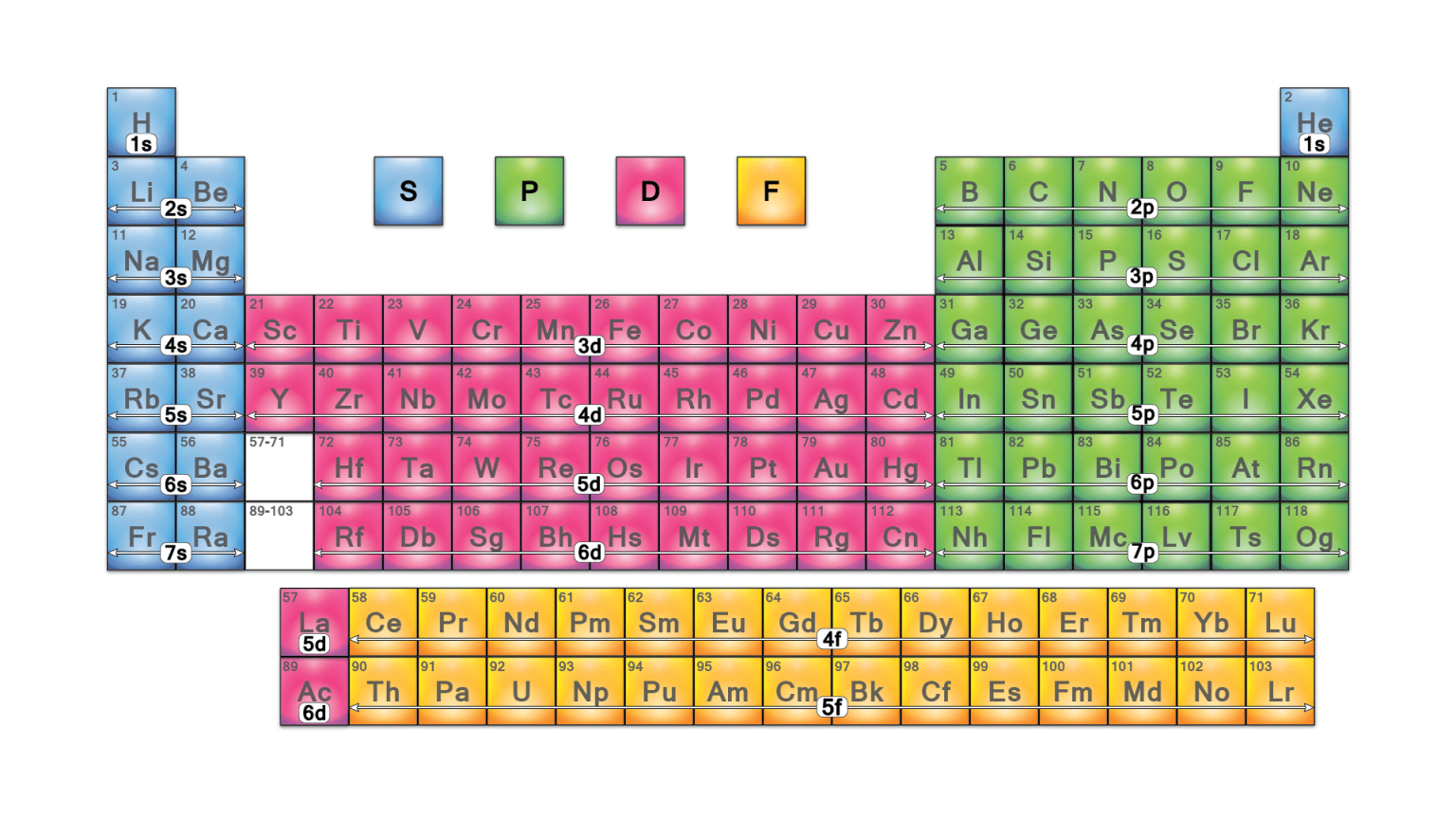
Periodic Table groups | Chemistry Nexus In the standard form of the periodic table the s-block, p-block, and d-block elements are organised into 18 vertical columns called groups. These are labelled from 1 to 18 under current IUPAC numenclature. Earlier labelling schemes (Trivial Group names) For historical reasons some Groups have special names. Terms such as the "alkali metals ... Ointment 0.03% Ointment 0.1% - Food and Drug Administration In clinical studies with periodic blood sampling, a similar distribution of tacrolimus blood levels was also observed in adult patients, with 90% (1253/1391) of patients having a blood concentration less than 2 ng/mL. The absolute bioavailability of tacrolimus from PROTOPIC in atopic dermatitis patients is approximately 0.5%. In adults with an average of 53% BSA … LABEL-AIRE 3115NV MANUAL Pdf Download | ManualsLib 4. Label advance occurs but Replace the control module or request qualified assistance. fails to stop or is erratic. (Cont.) Incorrect Label Repeat Distance. Refer to the set-up procedure and correct. 5. Label placement on the Replace the damaged roll of labels. Page 120: Label Placement On The Product Is Consistently Poor Labeling_the_Periodic_Table (1).pdf - Labeling the Periodic... Metalloids are found in groups3A-7A. They are Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium and Astatine. They are also called semi-metals. Color these all the same color. 5. Nonmetals a. Noble Gases (also called Inert Gases)b. Halogens (the halogens are the elements in group 7A)c. Nonmetals in groups 4A-6A d. Hydrogen 6.
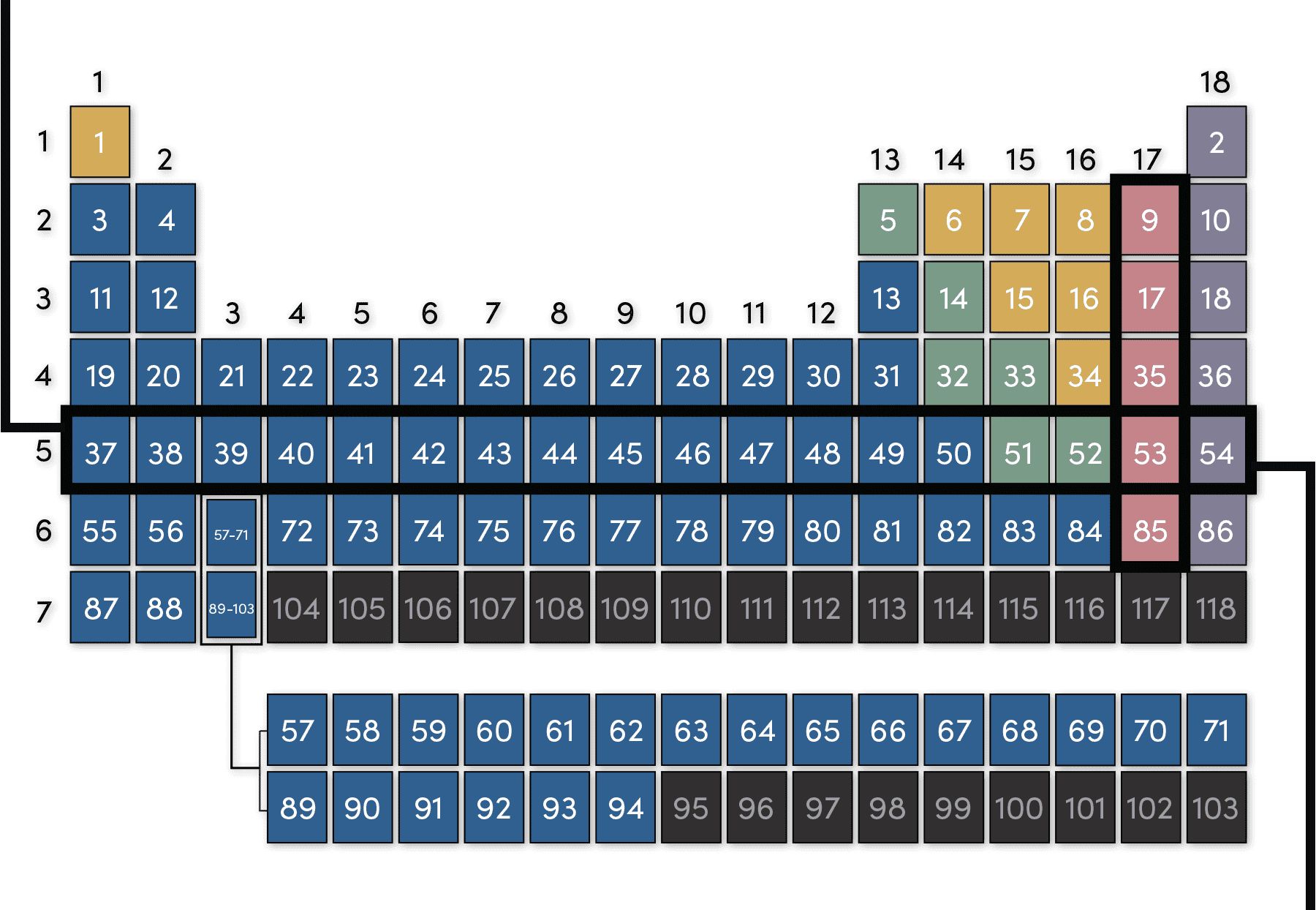
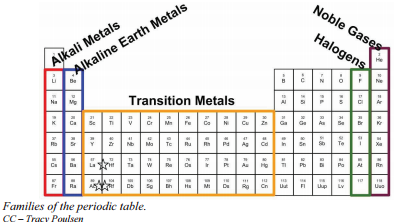
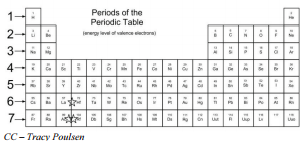
How to make a bulk upload spreadsheet for Business Profiles You can search for businesses by label from the dashboard, and use labels to filter location extensions in Google Ads. Assign up to 10 unique labels to each location. Labels can be up to 50 characters long and should not include invalid characters (i.e. < or >). To include commas in the label name, use the string "%2c" in your spreadsheet. For ... Groups and Periods in the Periodic Table - breakingatom.com Oxidation States of Ions and Atoms. Radioactivity and the Decay of Nuclei. Common Groups and Periods of the Periodic Table. Group 1: The Alkali Metals. Group 2: The Alkaline Earth Metals. The Transition Metals. Group 17: The Halogens. Group 18: The Noble Gases. The Periodic Table | Chemistry for Majors - Lumen Learning Groups are labeled at the top of each column. In the United States, the labels traditionally were Roman numerals with capital letters. However, IUPAC recommends that the numbers 1 through 18 be used, and these labels are more common. Grouping of Elements in the Periodic Table - Biochemistry Facts Halogens. The elements found in this Group are the top 4 elements of Group 17 which include Fluorine, Chlorine, Astatine and Iodine and they all represent the second subset of non metals. The Halogens are chemically reactive and tend to easily react with Akali metals in order to produce different types of salts.
Group (periodic table) - Wikipedia In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements.There are 18 numbered groups in the periodic table; the f-block columns (between groups 2 and 3) are not numbered. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms (i.e., the same core charge), because ...
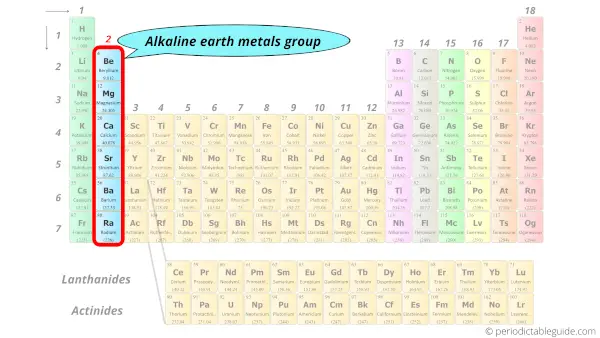
On Periodic Table Label Alkaline Earth Metals On Periodic Table Label Alkaline Earth Metals. Alkali alloys The name alkali precious metals arises from the Arabic phrase al-qali, meaning ashes. These metals are most reactive when in contact withwater and air, or oil. They may be usually found in salts, and also have a body-focused cubic construction.
Table of Elements and Chemistry Mendeleev found he could arrange the 65 elements then known in a grid or table so that each element had: 1. A higher atomic weight than the one on its left. For example, magnesium (atomic weight 24.3) is placed to the right of sodium (atomic weight 23.0): The True Basis of the Periodic Table. In 1913, chemistry and physics were topsy-turvy.
periodic table | Definition, Elements, Groups, Charges, Trends, & Facts ... periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number—i.e., the total number of protons in the atomic nucleus. When the chemical elements are thus arranged, there is a recurring pattern called the "periodic law" in their properties, in which elements in the same column (group) have ...
Periodic Table of Elements: Los Alamos National Laboratory Group. See About the Periodic Table for information on how Group can be used to characterize an element. Group 1: alkali metals, or lithium family. Group 2: alkaline earth metals, or beryllium family. Group 3: the scandium family. Group 4: the titanium family. Group 5: the vanadium family. Group 6: the chromium family.
List of Periodic Table Groups - ThoughtCo The noble gases, also known as the inert gases, are located in Group VIII of the periodic table. The noble gases are relatively nonreactive. This is because they have a complete valence shell. They have little tendency to gain or lose electrons. The noble gases have high ionization energies and negligible electronegativities.
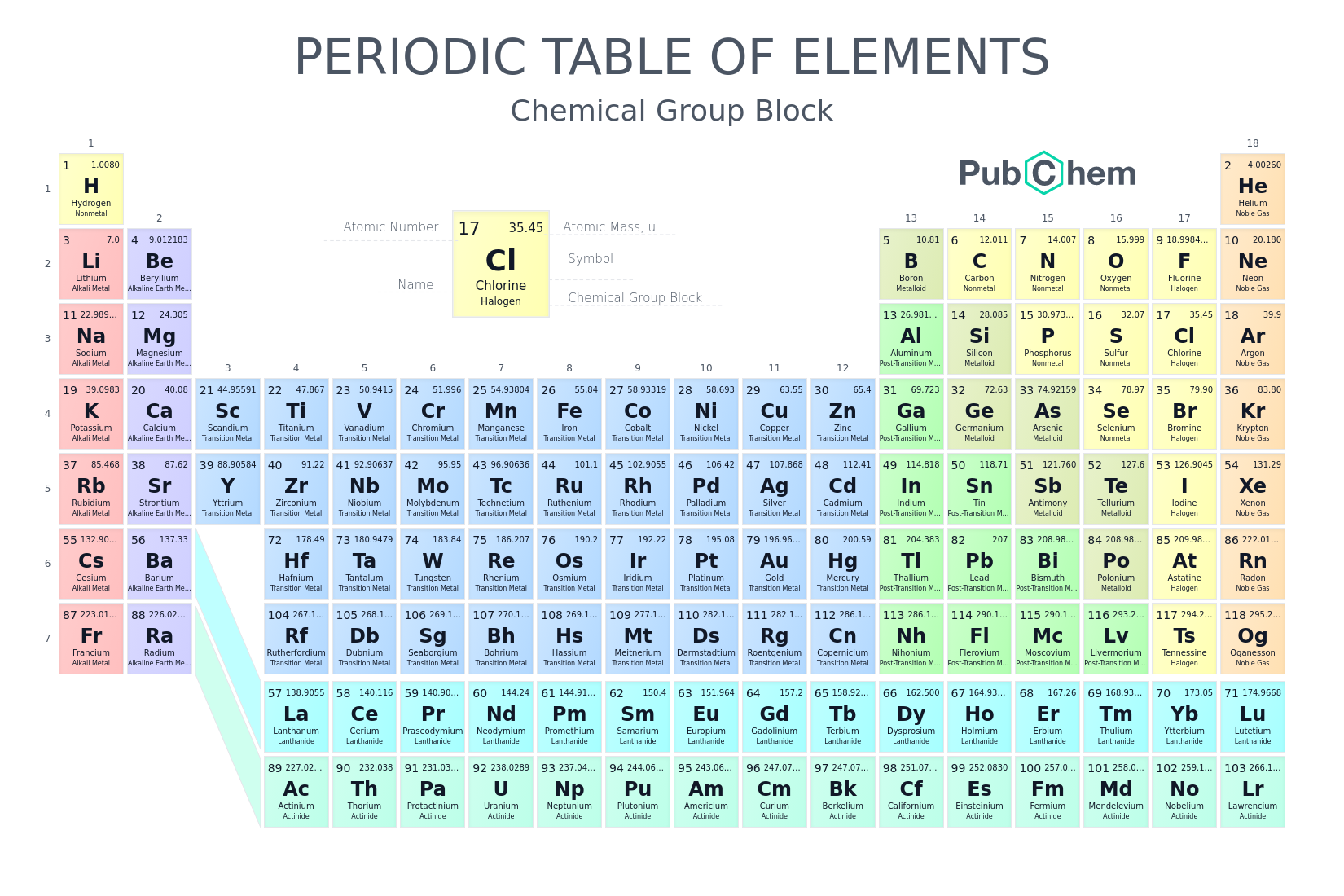
Periodic Table of Elements - PubChem Finally, IUPAC assigns collective names (lanthanoids and actinoids) and group numbering (1 to 18) and has investigated the membership of the group 3 elements. PubChem is working with IUPAC to help make information about the elements and the periodic table machine-readable.
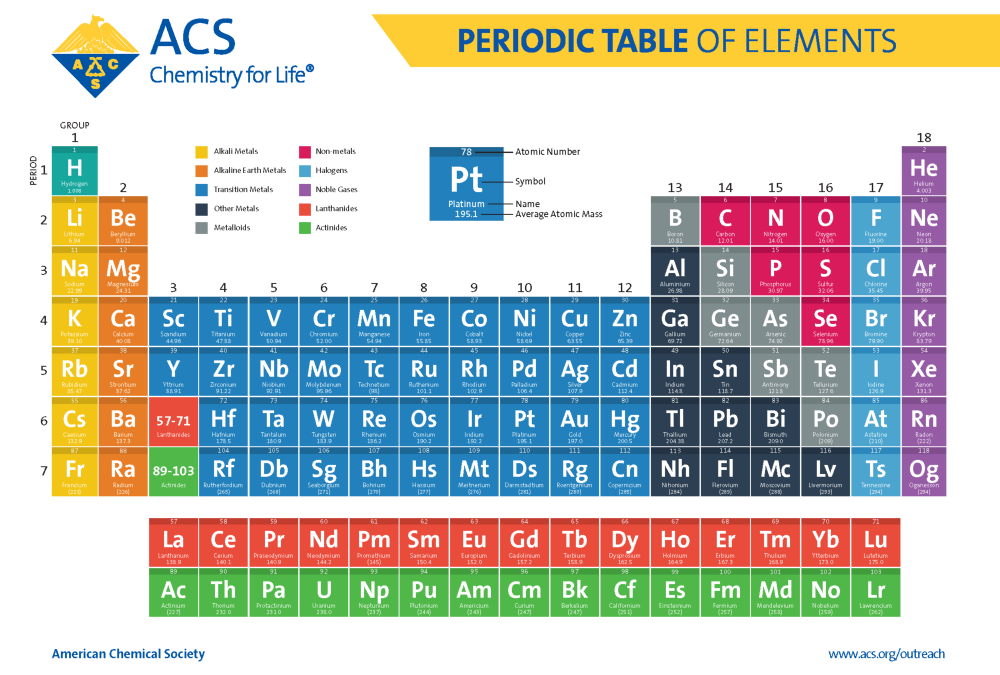
Standard periodic table, group labels - Stock Image - C001/3001 Well-known groups include the alkali metals (Group 1), the alkaline earth metals (Group 2), the halogens (Group 17) and the noble gases (Group 18). The standard periodic table has 118 elements arranged in 18 groups and 7 periods. Each element is represented by its chemical symbol. Above each symbol is the element's atomic number, and below it ...
OneClass: Label these groups of the periodic table. Rank from largest to smallest atomic radius. To rank items as equivalent, overlap them. The periodic table lists all known elements by their masses and groups elements by their properties. Within the periodic table, there are many properties of the elements that have periodic trends as we move down the groups in the table or across the periods.
chemicalelements.comChemical Elements.com - An Interactive Periodic Table of the ... Show Table With: Name Atomic Number Atomic Mass Electron Configuration Number of Neutrons Melting Point Boiling Point Date of Discovery Crystal Structure. Element Groups: Alkali Metals Alkaline Earth Metals Transition Metals Other Metals Metalloids Non-Metals Halogens Noble Gases Rare Earth Elements
. Periodic Table of the Elements, Calculators, and ... The periodic table is a chart that organizes the elements by increasing atomic number and their chemical and physical properties (see article History of the Periodic table of elements). Rows are arranged so that elements with similar properties fall into the same vertical columns ("groups").
The Periodic Table | CHEM 1305: General Chemistry I—Lecture - Course Hero Groups are labeled at the top of each column. In the United States, the labels traditionally were numerals with capital letters. However, IUPAC recommends that the numbers 1 through 18 be used, and these labels are more common.
Solved Label these groups of the periodic table. | Chegg.com Label these groups of the periodic table. Question: Label these groups of the periodic table. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use ...
Functional group - Wikipedia Table of common functional groups. The following is a list of common functional groups. In the formulas, the symbols R and R' usually denote an attached hydrogen, or a hydrocarbon side chain of any length, but may sometimes refer to any group of atoms.. Hydrocarbons. Hydrocarbons are a class of molecule that is defined by functional groups called hydrocarbyls …
Color of all the elements in the Periodic Table - SchoolMyKids In the below periodic table you can see the trend of . Color. For facts, physical properties, chemical properties, structure and atomic properties of the specific element, click on the element symbol in the below periodic table.Property Trends for Color. of the elements in the periodic table
Periodic Classification of Elements: Periodic Table - Embibe Groups: The vertical columns in a periodic table are called groups. There are \(18\) groups in the modern periodic table. 1. The elements in a particular group exhibit the same valency and similar chemical properties. The physical properties of the elements in the group vary gradually. 2. The number of the shell increases down the group. 3.
How to Find Valence Electrons: 12 Steps (with Pictures) - wikiHow 03.08.2022 · Label each column on the periodic table of elements from 1 to 18. Generally, on a periodic table, all of the elements in a single vertical column will have the same number of valence electrons. If your periodic table doesn't already have each column numbered, give each a number starting with 1 for the far left end and 18 for the far right end. In scientific terms, …
The Periodic Table: Families and Periods - dummies The vertical columns of elements are called groups, or families. The most common way the periodic table is classified is by metals, nonmetals, and metalloids . Periods in the periodic table In each period (horizontal row), the atomic numbers increase from left to right. The periods are numbered 1 through 7 on the left-hand side of the table.
Periodic table labeled with Metals Nonmetals and Metalloids Periodic table consists of total 118 elements. All these known elements are classified in three major categories based on their metallic and nonmetallic characteristics. Metals Nonmetals and Metalloids In the very beginning of this article, I showed you the Periodic table labeled with metals, Nonmetals and Metalloids.
EniG. Periodic Table of the Elements, Calculators, and Printable … Chemistry . Rare earth elements (REE) - Rare earth elements (REE) are a collection of seventeen chemical elements in the periodic table, specifically the fifteen lanthanides plus scandium and yttrium. History of the rare earth elements - The close chemical similarity of the rare earth elements is displayed in their occurring together in nature and further by the fact that it took …
How the Periodic Table groups the elements | Live Science The post-transition metals cluster to the lower left of this line. Metalloids: The metalloids are boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te) and polonium...
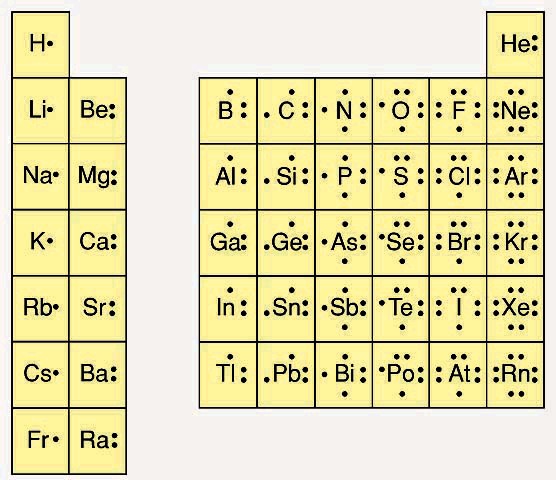
Labeled Periodic Table of Elements with Name [PDF & PNG] There are 18 groups in the periodic table, which consists of metal and nonmetal. Protons in the tables are positively charged particles. Neutrons are the neutrally negative charge, and electrons are the negative charge particles. It also shows the formation of a bond from one element to the other. PDF Labelled Periodic Table with Charges
study.com › academy › lessonMetals on the Periodic Table: Definition & Reactivity Oct 13, 2021 · This periodic table groups elements according to type: metal (blue), nonmetal (yellow), or metalloid (red). All of the metals are grouped together on the left side of the periodic table.
› periodic-table-of-elementProperties of Periodic Table of Element Groups - ThoughtCo This is what is meant by periodicity or periodic table trends . There are multiple ways of grouping the elements, but they are commonly divided into metals, semimetals (metalloids), and nonmetals. You'll find more specific groups, like transition metals, rare earths, alkali metals, alkaline earth, halogens, and noble gasses.
An Element In Group 2 Of The Periodic Table An Element In Group 2 Of The Periodic Table. Pauli Exclusion Basic principle The periodic law states that every compound elements possess a normal design of components. Mendeleev initial stated this rules in 1869, and the Pauli exclusion principle provided crucial theoretical help for this thought.
Periodic Table of Elements -Symbols, Atomic Number, Atomic Mass, Groups ... The table below consists of 118 elements of the periodic table, sorted by atomic number, atomic weight, symbols, density, discovered year and the group. Atomic Number of Elements There are about ninety elements found on Earth. Each one has a different number of protons, electrons and neutrons.
› Find-Valence-ElectronsHow to Find Valence Electrons: 12 Steps (with Pictures ... Aug 03, 2022 · Generally, on a periodic table, all of the elements in a single vertical column will have the same number of valence electrons. If your periodic table doesn't already have each column numbered, give each a number starting with 1 for the far left end and 18 for the far right end. In scientific terms, these columns are called the element "groups."
en.wikipedia.org › wiki › Functional_groupFunctional group - Wikipedia Table of common functional groups. The following is a list of common functional groups. In the formulas, the symbols R and R' usually denote an attached hydrogen, or a hydrocarbon side chain of any length, but may sometimes refer to any group of atoms.
Groups of the periodic table (video) | Khan Academy The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups. The elements in each group have the same number of valence electrons. As a result, elements in the same group often display similar properties and reactivity.
Periodic table Groups Explained !! (With 1-18 Group Names) Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li) Sodium (Na) Potassium (K) Rubidium (Rb) Cesium (Cs) Francium (Fr) For detailed information on Alkali metals, read the Ultimate guide on Alkali metals of periodic table.


:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)





:max_bytes(150000):strip_icc()/PeriodicTableCharge-WBG-56a12db23df78cf772682c37.png)













Post a Comment for "42 label these groups of the periodic table"