41 open label study
Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. › open-label-trialOpen-Label Trial - an overview | ScienceDirect Topics Open-label trials of desipramine, tranylcypromine, reboxetine, and bupropion showed improvement with the drug therapy. 55 An open-label trial of escitalopram for SAD patients (N = 20) over 8 weeks produced a response rate of 95% (SIGH-SAD < 50% of baseline value) and a remission rate (SIGH-SAD score < 8) of 85%. 56 In an open-label trial of duloxetine, a serotinin-norepinephrine reuptake inhibitor, response rates were 80.8% and remission rates were 76.9% (N = 26). 57 Agomelatine, a novel ...
Facebook - National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine.

Open label study
A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an ... Methods: We conducted an open-label, multicentre, controlled, cluster-randomised, crossover implementation study of a 12-gene pharmacogenetic panel in 18 hospitals, nine community health centres, and 28 community pharmacies in seven European countries (Austria, Greece, Italy, the Netherlands, Slovenia, Spain, and the UK). Patients aged 18 years ... en.wikipedia.org › wiki › Open-label_trialOpen-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] This contrasts with a double-blinded trial, where information is withheld both from the researchers and the participants to reduce bias. What is an open label extension study? • NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
Open label study. pubmed.ncbi.nlm.nih.gov › 17253876Open-label extension studies: do they provide meaningful ... Abstract. The number of open-label extension studies being performed has increased enormously in recent years. Often it is difficult to differentiate between these extension studies and the double-blind, controlled studies that preceded them. If undertaken primarily to gather more patient-years of exposure to the new drug in order to understand and gain confidence in its safety profile, open-label extension studies can play a useful and legitimate role in drug development and therapeutics. dian.wustl.edu › our-research › clinical-trialEnd of Trial and Open-Label Extension (OLE) Frequently Asked ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time. › content › 348What is an open label trial? | The BMJ May 23, 2014 · An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities. Effects of open-label placebos in clinical trials: a ... - Nature Open-label placebos (OLPs) are placebos without deception in the sense that patients know that they are receiving a placebo. The objective of our study is to systematically review and analyze...
Ciltacabtagene autoleucel, a B-cell maturation antigen-directed ... This single-arm, open-label, phase 1b/2 study done at 16 centres in the USA enrolled patients aged 18 years or older with a diagnosis of multiple myeloma and an Eastern Cooperative Oncology Group performance status score of 0 or 1, who received 3 or more previous lines of therapy or were double-refractory to a proteasome inhibitor and an immunomodulatory drug, and had received a proteasome ... An Open-Label Safety/Tolerability and PK Study With Azstarys® in ... How to Read a Study Record Study Description Go to Brief Summary: The is a multicenter, dose-optimized, open-label, safety/ tolerability and pharmacokinetic (PK) study with Azstarys® in children 4 and 5 years of age with attention-deficit/hyperactivity disorder (ADHD). › life-sciences › What-is-anWhat is an Open-Label Clinical Trial? - News-Medical.net Mar 31, 2022 · Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play a... Open-Label Trial - an overview | ScienceDirect Topics Open-label trials of desipramine, tranylcypromine, reboxetine, and bupropion showed improvement with the drug therapy.55 An open-label trial of escitalopram for SAD patients ( N = 20) over 8 weeks produced a response rate of 95% (SIGH-SAD < 50% of baseline value) and a remission rate (SIGH-SAD score < 8) of 85%. 56 In an open-label trial of dulo...
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology For example, in an open-label extension study of etanercept for ankylosing spondylitis, "efficacy analyses are reported without statistical inference between treatment groups"3. Although the CONSORT guideline has provided a frame-work for the design, analysis, and reporting of RCT4, no consensus statement has been developed for open-label Open Label Study: Treatment of ALS Fatigue With PolyMVA Study Description Go to Brief Summary: Amyotrophic lateral sclerosis (ALS) is a disease that causes the death of upper and lower motor neurons. ALS symptoms are characterized by stiffness, muscle twitching, and worsening weakness due to muscle breakdown. Open label extension studies and patient selection biases Concerns over the scientific validity of open label extension studies to randomized controlled trials have recently been raised. Patients experiencing adverse events will be withdrawn before the follow-on period of the study, and those experiencing milder side-effects will be less likely to opt to continue into the open label extension. Reducing bias in open-label trials where blinded outcome assessment is ... Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators aware of treatment allocation. Research has suggested that these trials should use blinded outcome assessment to avoid bias in estimated treatment effects [6-10]. Blinded outcome assessment is often ...
Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc.
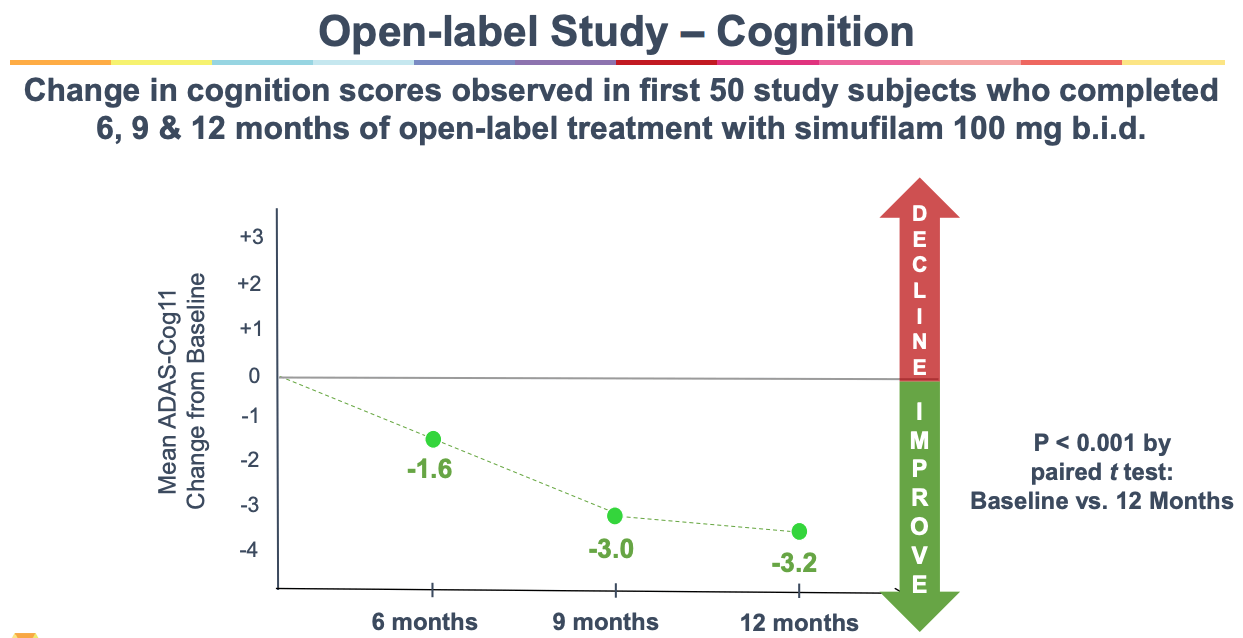
Simufilam (PTI-125), 100 mg, for Mild-to-moderate Alzheimer's Disease ... The study concludes with an additional 6-month open-label treatment period. Clinic visits are every month or month and a half in the first year, and every 3 months in the second year with an additional visit at Month 13. Cognition and neuropsychiatric symptoms are evaluated. Detailed Description:
Spotlight on Open-Label Extension Studies The stated objective of most OLE studies is to obtain long-term safety and tolerability data. Open-label extension (OLE) studies are common, but they do not receive as much attention as traditional Phase I through Phase IV studies. Enrollment into an OLE study typically follows enrollment into a randomized, blinded, well-controlled main study.
Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma ... This phase 3, open-label study compared the efficacy of isatuximab plus carfilzomib-dexamethasone versus carfilzomib-dexamethasone in patients with relapsed multiple myeloma. Methods. This was a prospective, randomised, open-label, parallel-group, phase 3 study done at 69 study centres in 16 countries across North America, South America ...
clinicalinfo.hiv.gov › en › glossaryOpen-Label Trial | NIH - HIV.gov Open-Label Trial. A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants.
What is an open label extension study? • NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
en.wikipedia.org › wiki › Open-label_trialOpen-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] This contrasts with a double-blinded trial, where information is withheld both from the researchers and the participants to reduce bias.
A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an ... Methods: We conducted an open-label, multicentre, controlled, cluster-randomised, crossover implementation study of a 12-gene pharmacogenetic panel in 18 hospitals, nine community health centres, and 28 community pharmacies in seven European countries (Austria, Greece, Italy, the Netherlands, Slovenia, Spain, and the UK). Patients aged 18 years ...






























![PDF] A phase II open-label, multicenter, study to evaluate ...](https://og.oa.mg/A%20phase%20II%20open-label,%20multicenter,%20study%20to%20evaluate%20the%20efficacy%20and%20safety%20of%20rivoceranib%20in%20subjects%20with%20recurrent%20or%20metastatic%20adenoid%20cystic%20carcinoma..png?author=%20Hyunseok%20Kang,%20Alan%20Loh%20Ho,%20Jameel%20Muzaffar,%20Daniel%20W.%20Bowles,%20Sung-Bae%20Kim,%20Myung-Ju%20Ahn,%20Glenn%20J.%20Hanna,%20Francis%20P.%20Worden,%20Tak%20Yun,%20Steven%20Norton,%20Neil%20Sankar,%20Bhumsuk%20Keam)
Post a Comment for "41 open label study"