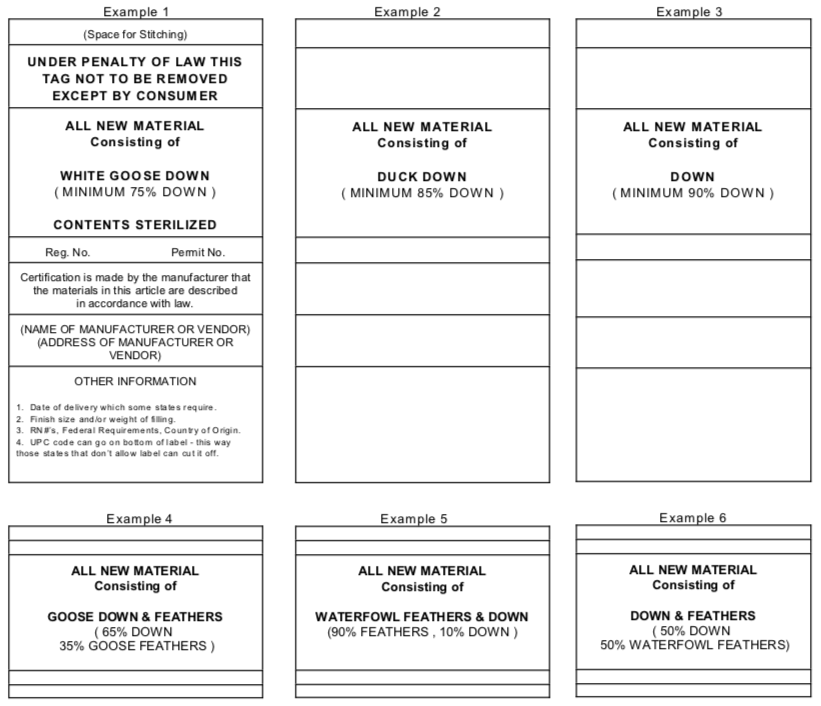
42 minimum information required for a manufacturers label
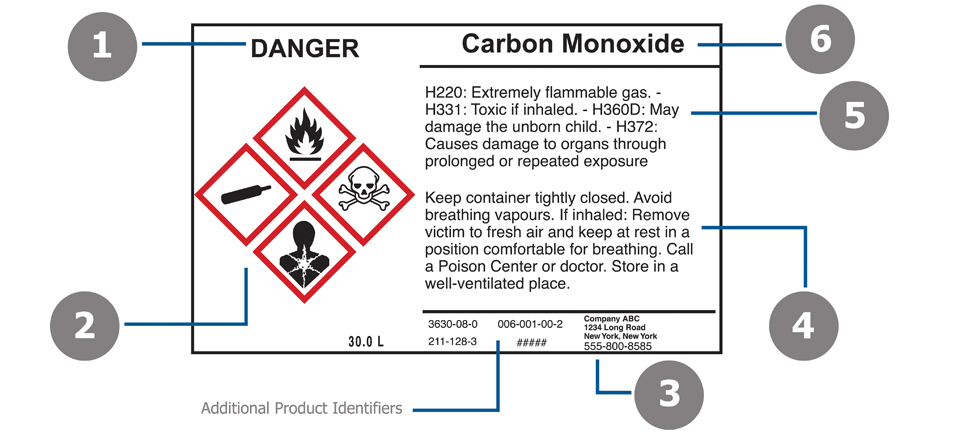
A Guide to OSHA's New GHS Chemical Labeling Requirements The GHS-inspired standards will require chemical manufacturers and importers to label chemical containers with 1) a harmonized signal word 2) GHS pictogram (s) 3) a hazard statement for each hazard class and category and 4) a precautionary statement. These elements are discussed in greater detail below: Quality System Regulation Labeling Requirements | FDA Labeling specifications are: engineering drawing and/or artwork for each label, appropriate inspection or control procedures, and appropriate procedures for attaching the labels. All procedures,...
Manufacturing Label Compliance: What you need to know. Application range = the temperature at which the product is labeled. Service range = the temperatures the product is stored at (or will encounter throughout the lifespan of the product). We have a full suite of label samples that use PolarBond for you to test for yourself. Send us an Email or give us a call at 888.972.5234. Heat and Moisture

Minimum information required for a manufacturers label
Chemical Container Labels | EHS - University of Washington Original manufacturer labels The label on an original chemical container must be legible and written in English. It must include the chemical/product name as shown on the SDS and the manufacturer's name and address. Do not accept materials if the label is illegible or missing required information. (See example of original label below). United States Product Labeling Requirements: An Overview - Compliance Gate Manufacturers or importers can choose to use a Registered Identification Number (RN) instead of their company name on the product label. Applicants are required to submit the following information to the FTC: Business email address Legal business name/company name Company address Company type (ie. LLC, partnership) PDF Number 384 - Revised January 2009 LABELING REQUIREMENTS FOR ... The ATCM specifies the minimum information required for a label but does not specify the format, color, size, or font for the label. These choices are left to the manufacturer or fabricator to allow flexibility to meet the needs of individual companies . All required information must be in readable English and not in code.
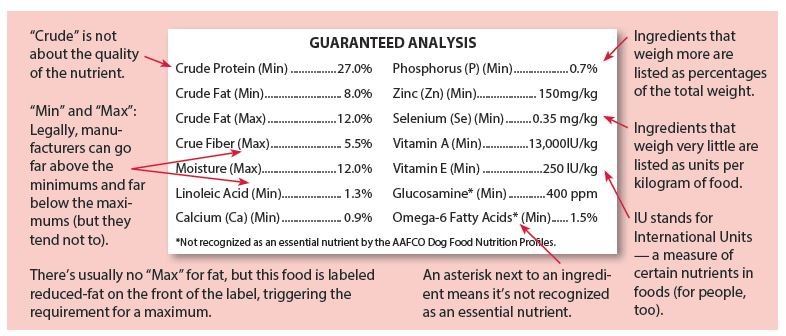
Minimum information required for a manufacturers label. What is the Minimum Information Required For An SDS? - Quantum Compliance Minimum Information for an SDS: Identification of the substance/mixture and of the supplier GHS Product Identifier Other means of identification Recommended use of the chemical and restrictions on use Supplier's details (name, address, phone number, etc) Emergency phone number Hazard Identification What Information Is Required On Secondary Container Labels? - XO Safety OSHA Requirements for Secondary Container Labels. OSHA requires secondary container labels to have the full GHS label, or: "Product identifier and words, pictures, symbols, or combination thereof, which provide at least general information regarding the hazards of the chemicals, and which, in conjunction with the other information immediately ... EU - Labeling/Marking Requirements - International Trade Administration CE Mark. CE marking is probably the most widely used and recognized marking required by the European Union. Found in all "New Approach" legislation with a few exceptions, the placement of the CE mark on a product serves as the manufacturer's declaration that the item meets all EU regulatory requirements (typically related to safety, health, energy efficiency, or environmental concerns ... What are the requirements for machinery CE marking plates? Any markings necessary if the machinery is designed and constructed for use in a potentially explosive atmosphere. Any other information relevant to the machine's type and that is essential for its safe use. Where a machine part must be handled during use with lifting equipment, its mass must be indicated.
Labels on ampoules 5mL or smaller | Occupational Safety and Health ... This practical accommodation requires the manufacturer to include, at a minimum, the following information on the label of the immediate container: Product identifier Appropriate pictograms Manufacturer's name and phone number Signal word A statement indicating the full label information for the chemical is provided on the outside package. PDF New OSHA Hazard Communication Standard -Minimum Training Requirements- -Minimum Training Requirements- Chemical manufacturers, importers, distributors, and employers must have employees trained . on the new label elements and the SDS format by December 1, 2013. This material is provided by Midwest Hardware Association and it may be used as a guide for training your employees on the new 5 Calibration Label Requirements to Meet Quality Certification ... 5 Label Requirements to Help Meet Certification Standards. To meet certification standards, labels and tags require direct traceability and must reference: The name of responsible body or organization using them. An I.D. number (i.e., serial number or other equipment identifier. This confirms certification for a specific piece of equipment ... General Device Labeling Requirements | FDA Name and Place of Business ( 21 CFR 801.1) The label of a device shall contain the name and place of business of manufacturer, packer, or distributor including the street address, city, state, and...
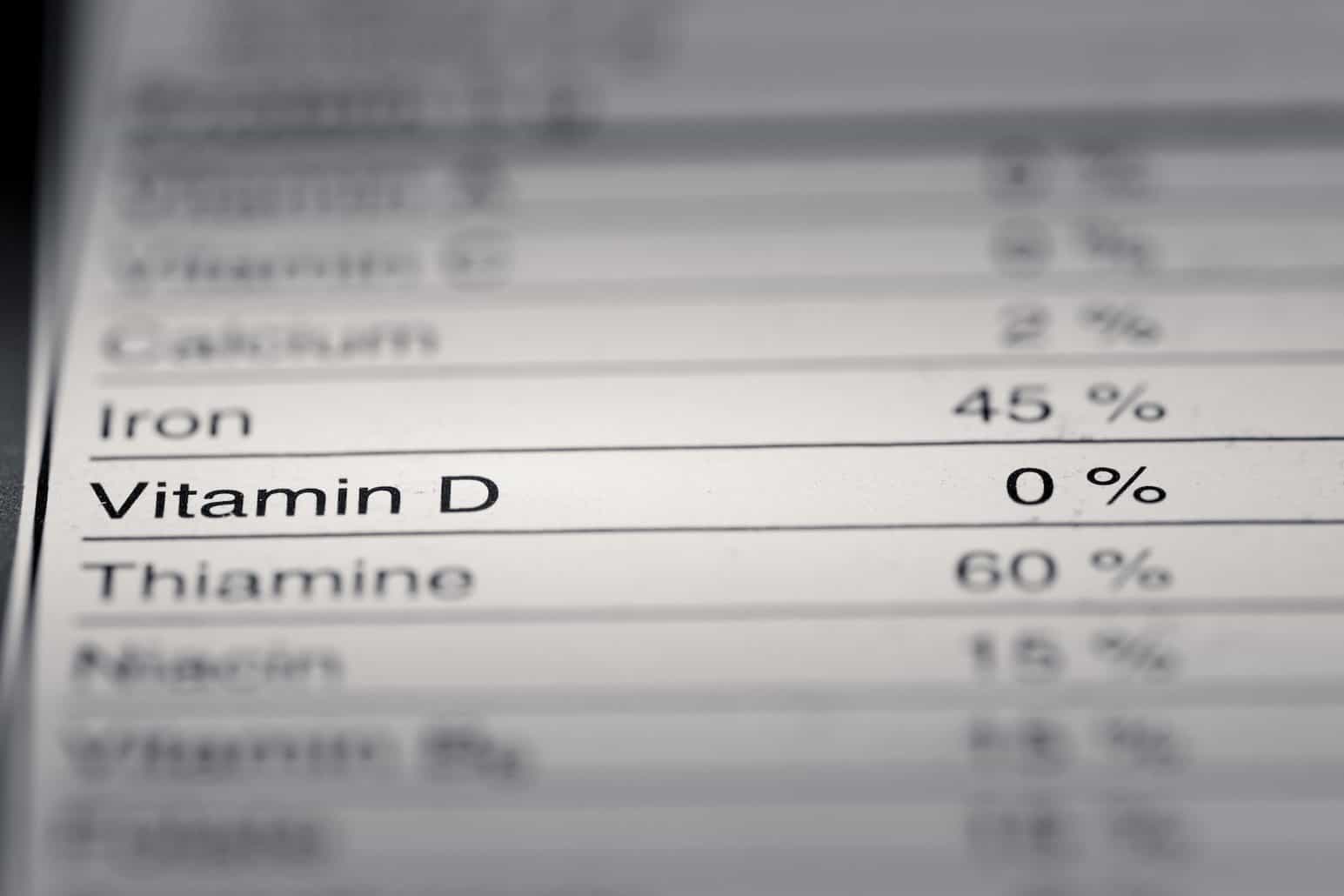
FDA Food Product Labeling & Packaging Requirements - ESHA Food Product Labeling and Packaging 101. The FDA regulates most packaged foods sold in the United States and has specific requirements for what elements a package must contain (e.g. a Nutrition Facts panel, ingredient statement, etc.). In order to sell your food products, you must comply with the FDA's packaging laws unless your operation is ... Chemical Safety: Labels and SDSs - Washington State University Every chemical container must be labeled by the manufacturer or supplier with general information on the potential hazards and how to use the product safely. The label at a minimum must have the: name of the product, name and address of the manufacturer, and the physical and health hazards associated with the product. GHS Labeling Requirements: The Definitive Guide [2021 Update ... - Luminer However, the GHS takes into consideration that, sometimes, it's not possible to keep workers safe with only these six label requirements, which is why it also allows for supplemental information. 1. Product Identifier This requirement identifies the actual hazardous chemical inside the container. Labeling Requirements | US EPA The label on a pesticide package or container and the accompanying instructions are a key part of pesticide regulation. The label provides critical information about how to handle and safely use the pesticide product and avoid harm to human health and the environment. Labeling Requirement Resources
What Required Information Must GHS Labels Include? - MPC Manufacturer information - GHS labels must include the manufacturer's name, as well as contact information including an address and phone number.
eCFR :: 9 CFR 317.2 -- Labels: definition; required features. (b) Any word, statement, or other information required by this part to appear on the label must be prominently placed thereon with such conspicuousness (as compared with other words, statements, designs, or devices, in the labeling) and in such terms as to render it likely to be read and understood by the ordinary individual under customary conditions of purchase and use. In order to meet this ...
Garment Labelling Requirements for Clothing (Full Guide) A garment label on a textile product sold in the USA must feature the registered identification number (RN) of the manufacturer, importer, or corporate entity handling the sale of the product. All domestic textile companies and importers are required to have RNs.
Labeling Requirements for Commercial and Industrial Equipment At a minimum, such labels must include the energy efficiency of the equipment to which the rulemaking applies, as tested under the prescribed DOE test procedure. Such rule may also require the disclosure of estimated annual operating costs and energy use determined in accordance with the prescribed DOE test procedure.
General Guidelines for Labeling and Other Information Required to Be ... The product identification (labeling) and compliance information requirements for a device subject to SDoC (Sections 2.1074 and 2.1077, respectively) requires that each device be uniquely identified (for example, using a label listing a trade name and type or model number),4and that end-users must be 1See 47 CFR §§ 15.19 and 15.105.
Compliance FAQs: Packaging and Labeling in the US | NIST The UPLR requires that consumer packaging bear a label specifying the following: the identity of the commodity the name and place of business of the manufacturer, packer, or distributor the net quantity of contents in terms of weight or mass measure, or numerical count in a uniform location upon the principal display panel.
Manufacturer's name and address on the label of a chemical product ... Therefore, the address required by 29 CFR 1910.1200 (f) (1) (vi) is the physical or mailing address for the manufacturer, importer or distributor. The web address for your company may be included on the label as supplemental information.
How to properly label a medical device according to the MDR (2017/745)? In order to correctly label the medical device, the manufacturer should provide the fallowing information on the packaging / label of the device: name or trade name of the product, an indication that the device is a medical device [or a harmonized symbol] CE mark with the number of the notified body (if applicable), manufacturer's name and ...
Pharmaceutical Labeling: Requirements & Guidelines The label must remain in place on the container and be legible across its lifespan, including distribution, storage and use. The printing on the label must also be legible across this lifespan. (This means you need to use labels that have strong adhesive strength and are resistant to water and UV light.)
eCFR :: 21 CFR Part 201 -- Labeling (c) The labeling required by §§ 201.57 and 201.100(d) for sulfite-containing epinephrine for injection for use in allergic emergency situations shall bear the warning statement "Epinephrine is the preferred treatment for serious allergic or other emergency situations even though this product contains (insert the name of the sulfite, e.g ...
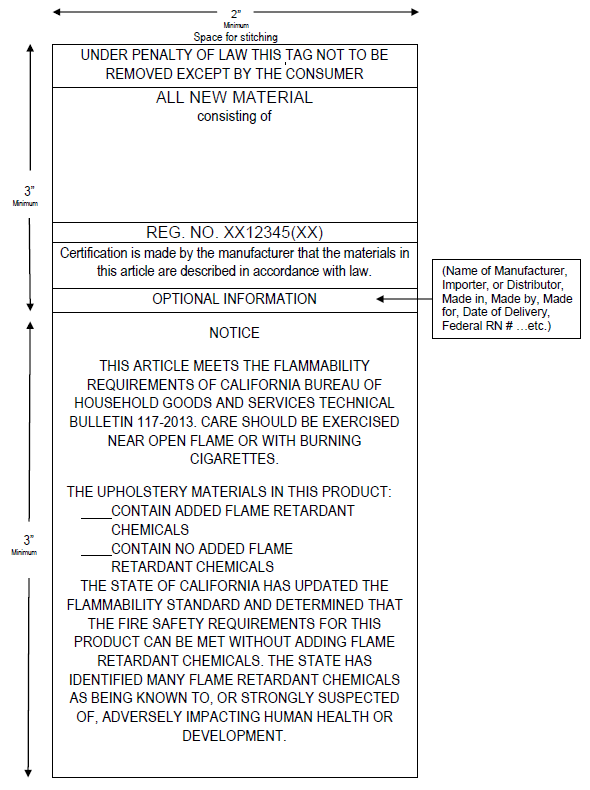
PDF Number 384 - Revised January 2009 LABELING REQUIREMENTS FOR ... The ATCM specifies the minimum information required for a label but does not specify the format, color, size, or font for the label. These choices are left to the manufacturer or fabricator to allow flexibility to meet the needs of individual companies . All required information must be in readable English and not in code.
United States Product Labeling Requirements: An Overview - Compliance Gate Manufacturers or importers can choose to use a Registered Identification Number (RN) instead of their company name on the product label. Applicants are required to submit the following information to the FTC: Business email address Legal business name/company name Company address Company type (ie. LLC, partnership)
Chemical Container Labels | EHS - University of Washington Original manufacturer labels The label on an original chemical container must be legible and written in English. It must include the chemical/product name as shown on the SDS and the manufacturer's name and address. Do not accept materials if the label is illegible or missing required information. (See example of original label below).










![Infographic] The importance of Sample Management in New ...](https://selerant.com/hubfs/Glossary%20Feature%20Images/Sampling_Infographic_Glossary_01.png)
















Post a Comment for "42 minimum information required for a manufacturers label"